Highlighted
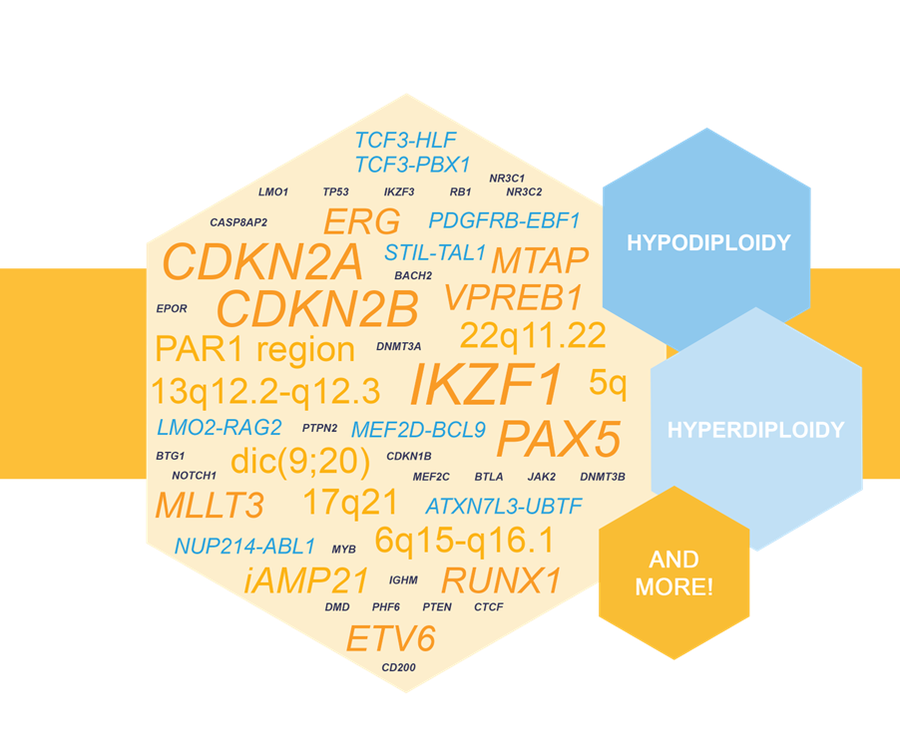
NXtec D007 Acute Lymphoblastic Leukemia as a Valuable Tool in pALL Testing
D007 Acute Lymphoblastic Leukemia was recognized as a valuable tool for enhanced molecular testing of pediatric acute lympoblastic leukemia (pALL) in a recent research article.

Holiday Closure & Shipping Information
MRC Holland will be closed from December 25, 2025 until January 1, 2026.

New Product: NXtec D024 KaryoProfiler — High-Throughput, High-Resolution Alternative to Traditional Karyotyping
We have launched NXtec D024 KaryoProfiler, our newest digitalMLPA™ assay designed for comprehensive molecular karyotyping across a wide range of applications.


