Principle of MLPA
The principle of SALSA® MLPA® is explained in the video below. Read on for a textual explanation. For alternative ways to view the video, see this support article.
MLPA probes
MLPA probemixes have probes that target a specific genomic sequence. An MLPA probe consists of two parts: a left and a right probe oligonucleotide (LPO and RPO). The LPO and RPO contain PCR primer and DNA hybridisation sequences. An additional stuffer sequence in the RPO gives each probe a unique length.

Sample denaturation and probe hybridisation
In the first step, purified sample DNA is denatured. This is followed by overnight incubation with MLPA probe oligos. The LPO and RPO parts of each probe hybridise to immediately adjacent target DNA sequences.
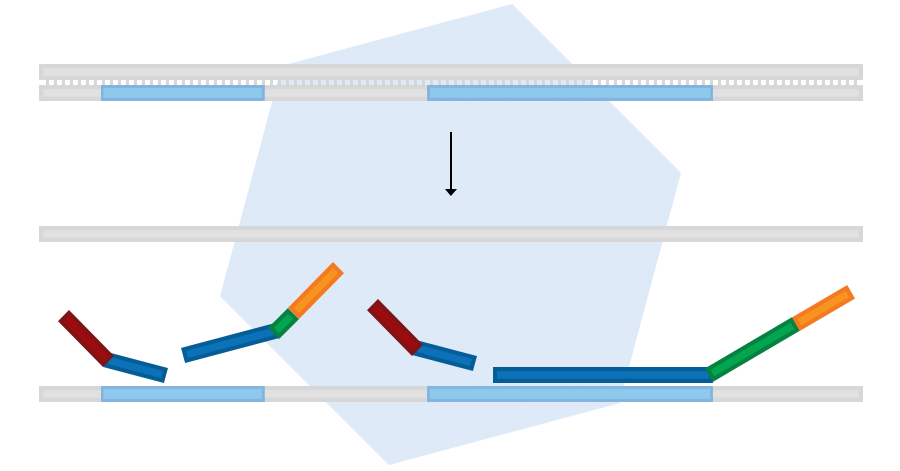
Probe ligation
The second step is the ligation of probe oligos that hybridised to immediately adjacent target sequences. No mismatches around the ligation site are permitted, making the ligation reaction highly specific. The number of probe ligation products is a measure for the number of target sequences in the sample.

Probe amplification
In the third step, ligated probes are amplified in a multiplex PCR using a single universal primer pair. Only ligated probes are exponentially amplified, making the removal of unbound and non-ligated probes unnecessary.

Fragment separation
The fourth step is fragment separation by capillary electrophoresis. PCR products are loaded onto a capillary electrophoresis device and separated by length. Each fragment corresponds to a specific MLPA probe.

Data analysis
The final step is data analysis by Coffalyser.Net™. Relative copy numbers are determined by comparing the relative peak heights of reference probes and target probes in the test samples with those in reference samples with a known normal copy number. Advanced quality checks help to recognize unreliable data.

More Information
Read our MLPA General Protocol.
The MLPA technique was first described in Schouten et al. 2002.