News Archive
Changes to Our Product Packaging
Our product packaging will receive updates which simplify processing and pave the way for future innovations – there are no changes to the product contents.
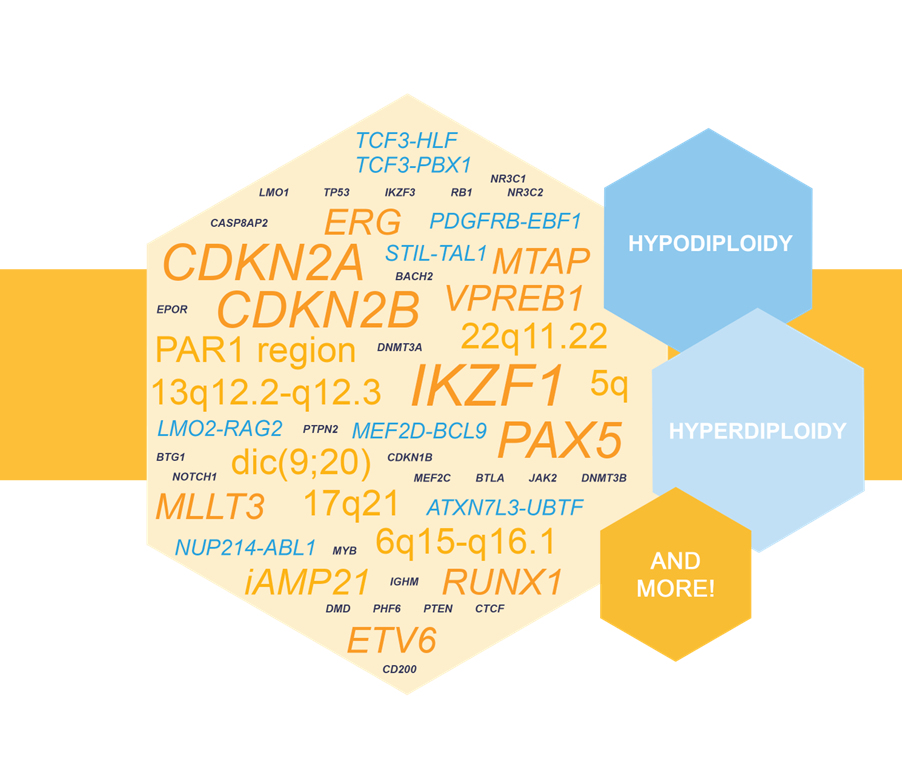
NXtec D007 Acute Lymphoblastic Leukemia as a Valuable Tool in pALL Testing
D007 Acute Lymphoblastic Leukemia was recognized as a valuable tool for enhanced molecular testing of pediatric acute lympoblastic leukemia (pALL) in a recent research article.
Our Products are IVDR-Certified!
We have successfully obtained our certification under the new In Vitro Diagnostic Regulation (IVDR; 2017/746)! The first batch of flagship products are already available on the market under the new IVDR, well in advance of the transition period deadline.
Holiday Closure & Shipping Information
MRC Holland will be closed from December 25, 2025 until January 1, 2026.
New Product: NXtec D024 KaryoProfiler — High-Throughput, High-Resolution Alternative to Traditional Karyotyping
We have launched NXtec D024 KaryoProfiler, our newest digitalMLPA™ assay designed for comprehensive molecular karyotyping across a wide range of applications.
New Product Launch: NXtec D002 Hereditary Cancer Panel 2 for Broad Hereditary Cancer Analysis
Introducing NXtec Hereditary Cancer Panel 2 – a broad panel for the detection of CNVs in cancer-associated genes and the presence of selected variants.
Improved – P242 Pancreatitis – Now Featuring Mutation-Specific Probes for SPINK1 and PRSS1
P242 Pancreatitis just received a major update, with the recently released D1 version featuring nine new target probes and three new mutation-specific probes, as well as three replaced target probes and eight replaced reference probes. The updated probe content facilitates enhanced detection of HP-related genetic alterations and improves assay robustness.
MRC Holland Visiting Scientist to Present on Newborn Screening at International Conferences
Terence Diane Fabella, a PhD candidate and MRC Holland researcher, will present her work on the development of digitalMLPA assays for newborn screening at three international conferences later this year.
MCR Holland Prices in 2026
Effective from 1 January 2026, we will be implementing a price increase of 3.6% on average on most of our products.
Unlocking CNV Certainty: What We Shared at ESHG 2025
We were delighted to attend the 2025 edition of the European Human Genetics Conference in Milan, Italy - read further for our re-cap.